Luna’s Sprayable Adhesion Barrier Hydrogel to be presented at the Society for Biomaterials Annual Meeting
Lauren Costella, Research Specialist
Christopher Tison, PhD, Team Leader
Biomedical Technologies Group
Postoperative Adhesions Prevention Remains a Critical Problem
Adhesions typically form following surgery when normal wound healing is interrupted by contact between injured and neighboring tissues. This results in the formation of fibrous connections that permanently attach neighboring tissues and typically causes pain, bowel obstruction, infertility, and increased difficulty in subsequent surgical procedures. With 44% of the population requiring some form of abdominal surgery prior to the age of 60, and 90% of all patients (including those receiving current treatments) reporting of abdominal procedures intra-abdominal complications as a result of adhesions, this is a significant problem that requires a novel solution.
The current standard of care for adhesion prevention is improved surgical technique and the application of a solid barrier film, such as SepraFilm™ (Genzyme). Seprafilm is reportedly difficult to handle and, while successful in preventing adhesions that form to the abdominal wall, fails to effectively protect other internal tissue surfaces that may have been handled. Other approaches using liquid infusion techniques are too quickly resorbed and fail to provide protection during the 3-5 day window in which adhesions typically form.
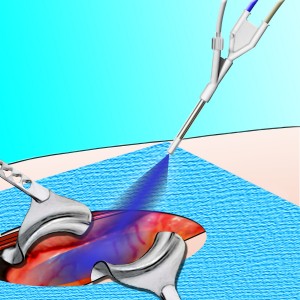
TissuCoat™ is a Unique Sprayable Product to Protect Injured Surfaces
为了防止粘连,并提供一个n easy-to-use, low-cost, effective solution to this market need, Luna is developing TissuCoat™ - a two component system in which biopolymer solutions are mixed upon application to form a thin barrier on the tissue surface. The resultant hydrogel is colored blue to enable visualization of coverage during application, and can be easily applied over irregular tissue surfaces and shapes to provide full coverage of all potential adhesion sites. This product is biodegradable, biocompatible, and adheres to the tissue surface. It is able to protect the injured tissue surface for 7-14 days as it is slowly resorbed and cleared from the body.
Luna has demonstrated the efficacy of this product as compared to controls using a small animal adhesion model in collaboration with the Department of Plastic Surgery at the University of Virginia. Results shown below demonstrated that Luna’s TissuCoat was able to significantly reduce the adhesion score for the two tissue surfaces the model is designed to investigate. Further, the total number of adhesions found on all tissue surfaces was also significantly reduced as compared to non-treated controls.
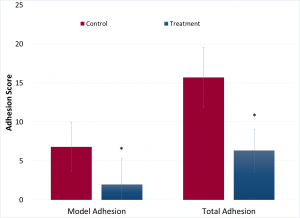
These preliminary results suggest that Luna’s TissuCoat has the potential to dramatically improve patient outcomes following surgery. Luna is now preparing to begin larger preclinical models using the rabbit uterine horn abrasion model with Charles River Laboratory, and is conducting in-depth biocompatibility confirmation studies with in collaboration with partners at North American Science Associates Inc. (NAMSA).
Presentation Details:
Luna will be presenting its TissuCoat™ development efforts during the upcomingSociety for Biomaterials Annual Meeting, taking place in Minneapolis, Minnesota from April 5-8. Luna will be presentingin vitroresults and small animalin vivostudies, along with a discussion of upcoming preclinical studies. This podium presentation is scheduled for Friday, April 7, at 4:45 pm in the *BTI* Commercialization of Biomaterials and Medical Products session.
Luna will also be presenting two poster at the Poster Sessions on Thursday, April 6 (6:00 pm) and Friday, April 7 (2:00 pm). The first poster, entitled “Biomimetic Materials for Ocular Surface Repair”, focuses on Luna’snanofiber-reinforced hydrogel bandagesfor ocular surface stabilization and regeneration. The second poster, entitled “Nanofiber Based Materials for Neural Interfacing” is focused on Luna’s efforts to develop a novel material system capable of matching the mechanical properties of peripheral nerve tissue.